Abstract

Introduction: Studies have suggested that 5% of adult patients with MDS and AML have inherited mutant alleles that predispose to disease development. More than 50 hereditary cancer syndromes have been defined-many specifically associated with myeloid and lymphoid malignancies. Yet hereditary hematologic malignancies (HHMs) continue to go unrecognized. The implications for patients, especially regarding family donor selection for allogeneic stem cell transplant, necessitate greater awareness. Referrals for genetic counseling at SKCC have been triggered primarily by a personal or family history of solid tumors. The aim of this study was to assess the frequency of hematologic malignancies in the setting of concurrent solid tumor diagnosis or significant family history of cancer.
Methods: In order to gain preliminary insight into the unmet need for genetic counseling for HHMs at our institution, we queried our Cancer Genetics REDCap database (IRB-approved #16D.743) for all patients who had a cancer diagnosis, a family history of cancer, and a hematologic malignancy (HM). Cancer diagnoses and family history were collected at time of genetic counseling by a genetic counselor and were primarily patient reported. After identifying patients with self-reported HMs, HM diagnoses were confirmed by medical history and pathology report, if available, in our electronic medical record system.
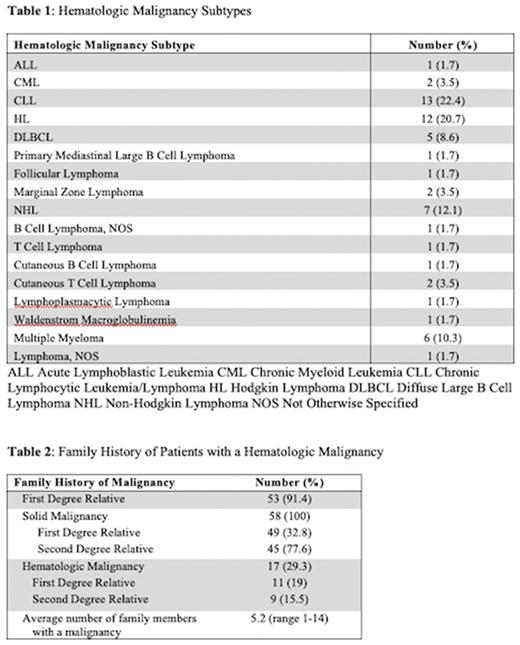
Results: The database included 5073 patients who were evaluated at SKCC's Cancer Genetics Program between 2014 and 2021. 3150 patients had a known cancer diagnosis including: breast (1812), prostate (349), ovarian (219), colon/rectal (218), pancreatic (157), melanoma (123), kidney (83), lung (18), and other (708). 4734 patients had a family history of cancer. 2913 patients had both a personal cancer diagnosis and a family history of cancer. Overall, 58 (2%) of these patients had a hematologic malignancy. Among patients with a HM, average age at HM diagnosis was 47 years (median 50, range 5-80) and average age at time of genetics counseling was 60 years (median 63.5, range 35-83). 48 of the patients were female. 51 were White/Caucasian, 6 were Black/African American, and 1 was Asian. 1 patient was Hispanic and 8 patients were Ashkenazi Jewish. There was a wide range of HM diagnoses (Table 1). 39 (67%) patients had a concurrent solid malignancy.
The 58 patients with a HM and family history of cancer had on average 5.2 family members with a malignancy (median 4, range 1-14). 53 (91.4%) of the patients had at least 1 first degree relative with a malignancy (Table 2). 17 (29%) patients had a family member with a known HM, including 11 (19%) with a first degree relative. HMs identified among first and second degree relatives included: CLL (N=5) Non-Hodgkin lymphoma (N=3), Hodgkin lymphoma (N=3), Waldenstrom macroglobulinemia (N=2), lymphoma not otherwise specified (N=3), AML (N=1), leukemia NOS (N=3), multiple myeloma (N=2), and polycythemia transformed to myelofibrosis (N=1).
Of the 40 HM patients who had germline genetic testing performed, alterations were identified in 22. 10 of the patients had 11 pathogenic or likely pathogenic variants in CHEK2, ATM, FANCC, NBN, MSH2, MSH6, BRCA2, and/or FH. 6 (60%) of these patients had alterations in genes known to be associated with HHMs. 21 variants of unknown significance were identified in 14 patients, including in 2 patients with pathogenic variants.
Conclusions: Approximately 2% of patients referred for genetic counseling for a personal malignancy and family history of cancer had a concurrent diagnosis of a HM. This likely under-represents the number of patients with HHMs, particularly since 6 of the 10 patients identified to have pathogenic or likely pathogenic variants had alterations in genes associated with HHMs. The findings that 67% of the HM patients had a second malignancy and that HM patients had on average 5 family members with cancer highlight the importance of identifying high risk patients to guide surveillance for earlier detection. Earlier recognition is crucial, as the average age of genetics counseling was 60. Despite striking family cancer histories, germline variants were not identified in several patients, suggesting that there may be germline variants that have yet to be identified. Efforts to expand genetic screening that focus on earlier identification, standardized germline testing for high risk patients, and appropriate surveillance will be important.
Disclosures
Palmisiano:AbbVie: Research Funding; Genentech: Research Funding. Porcu:Morphosys: Membership on an entity's Board of Directors or advisory committees; Innate Pharma: Honoraria, Membership on an entity's Board of Directors or advisory committees; Daiichi, Kyowa: Honoraria, Membership on an entity's Board of Directors or advisory committees; BeiGene: Membership on an entity's Board of Directors or advisory committees; Loxo: Membership on an entity's Board of Directors or advisory committees; DrenBio: Consultancy; Ono: Membership on an entity's Board of Directors or advisory committees; Teva: Honoraria, Research Funding; ADCT: Membership on an entity's Board of Directors or advisory committees; Viracta: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Kasner:Kartos/Telios: Research Funding; Astellas: Membership on an entity's Board of Directors or advisory committees; Gilead: Research Funding; Otsuka: Research Funding; Ono: Research Funding; BMS: Membership on an entity's Board of Directors or advisory committees; Kronos Bio: Other: Data and Safety Monitoring Board; Pfizer: Research Funding.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal